Zollinger-Ellison Syndrome Diagnostic Calculator
Calculate diagnostic probability based on fasting gastrin levels, PPI use, and clinical context. Follows guidelines from the article on pathophysiology and diagnosis.
Key Requirements: Stop proton pump inhibitors (PPIs) for 2 weeks prior to testing. Gastrin >1000 pg/mL is highly suggestive but requires confirmatory testing.
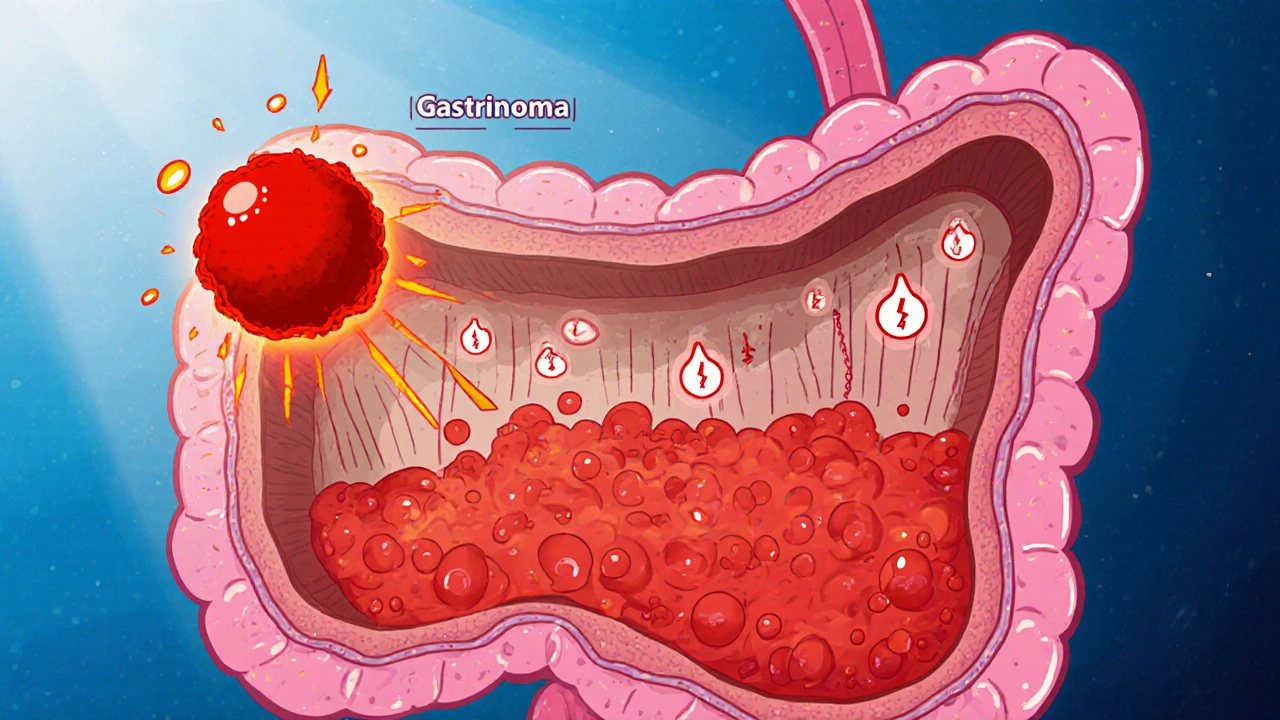
Zollinger-Ellison syndrome is a rare condition caused by gastrin‑secreting tumors (gastrinomas) that lead to excessive stomach acid production. The disease sits at the intersection of endocrine oncology and gastroenterology, making its underlying mechanisms both fascinating and clinically critical.
What Triggers the Acid Surge?
The first clue lies in the hormone Gastrin. Normally released by G‑cells in the antrum, gastrin tells the parietal cells to secrete hydrochloric acid. In Zollinger‑Ellison syndrome, a gastrinoma releases gastrin autonomously, ignoring normal feedback loops. This results in hypergastrinemia-the blood level of gastrin can be three to ten times higher than the upper reference limit.
Cellular Origin of Gastrinomas
Gastrinomas arise from neuroendocrine cells that have diverged from the usual G‑cell lineage. Most tumors develop in the duodenum (about 60 % of cases) or the pancreas (roughly 25 %). The remaining 15 % appear in the lymph nodes or elsewhere in the abdomen. Their neuroendocrine origin explains why they express markers such as chromogranin A and synaptophysin, which pathologists use to confirm the diagnosis.
Genetic Landscape: When MEN1 Enters the Picture
About 20‑30 % of patients carry a germline mutation in the MEN1 gene, which encodes the tumor suppressor protein menin. Loss‑of‑function mutations disrupt cell‑cycle regulation, predisposing carriers to multiple endocrine neoplasia type 1 (MEN1) syndrome. In this context, Zollinger‑Ellison syndrome often co‑exists with parathyroid hyperplasia and pituitary adenomas, forming a classic triad.
Signal Cascades That Amplify Acid Production
When gastrin binds to the CCK‑B receptor on parietal cells, it activates the phospholipase C (PLC) pathway, increasing intracellular calcium and stimulating the H⁺/K⁺‑ATPase proton pump. The downstream activation of the ERK1/2 MAPK cascade further enhances parietal cell proliferation. Chronic overstimulation can cause hypertrophy of the acid‑secreting cells, magnifying the already high acid output.
Clinical Fallout: From Ulcers to Malabsorption
The relentless acid barrage erodes the duodenal mucosa, leading to refractory peptic ulcers that often occur beyond the duodenal bulb. High acidity also inactivates pancreatic enzymes and damages the mucosal barrier, precipitating steatorrhea and nutrient malabsorption. Patients may present with abdominal pain, diarrhea, and weight loss, sometimes mistaken for Crohn’s disease.
Diagnosing the Hormonal Storm
Accurate diagnosis hinges on biochemical and imaging studies. The key laboratory test is a fasting gastrin level, but values can be misleading if the patient is on proton pump inhibitors (PPIs). The secretin stimulation test remains the gold standard: an abnormal rise in gastrin after secretin infusion confirms a gastrinoma.
| Test | What It Measures | Sensitivity | Typical Use |
|---|---|---|---|
| Fasting Gastrin | Baseline gastrin level | 70‑80 % | Initial screening (avoid PPIs 2 weeks prior) |
| Secretin Stimulation | Gastrin response to secretin | 90‑95 % | Confirmatory test when fasting gastrin is equivocal |
| Endoscopic Ultrasound (EUS) | Tumor size & location | 85‑90 % | Detect small duodenal gastrinomas |
| CT Scan (triple‑phase) | Cross‑sectional imaging of pancreas & liver | 75‑80 % | Staging and metastasis assessment |
| MRI with DWI | Soft‑tissue contrast, liver lesions | 80‑85 % | Alternative to CT when radiation avoidance is needed |
Therapeutic Targets: Controlling Acid and Tumor Growth
High‑dose proton pump inhibitors (PPIs) are the first line for acid suppression, often requiring doses 2‑4 times the standard regimen. For tumor control, surgical resection is curative in localized disease. When surgery isn’t feasible, somatostatin analogues (e.g., octreotide) inhibit gastrin release, while targeted agents like everolimus address the underlying neuroendocrine growth pathways.

Practical Checklist for Clinicians
- Stop PPIs at least 2 weeks before measuring fasting gastrin.
- Order secretin stimulation test if gastrin >1000 pg/mL or if clinical suspicion remains high.
- Use EUS for duodenal lesions under 2 cm; supplement with triple‑phase CT for pancreatic and hepatic assessment.
- Initiate high‑dose PPIs promptly to protect the mucosa while planning definitive therapy.
- Screen MEN1 gene in patients < 40 years or with a family history of endocrine tumors.
Common Pitfalls and How to Avoid Them
Misinterpretation of gastrin levels is a frequent error-elevated gastrin can also result from chronic atrophic gastritis or renal failure. Always correlate labs with clinical context and imaging. Another trap is under‑dosing PPIs; inadequate acid control can lead to persistent ulcers and bleeding. Finally, forgetting to assess for MEN1 can miss the chance for early surveillance of other endocrine neoplasms.
Future Directions in Research
Emerging data suggest that targeting the menin‑MLL interaction may curb gastrinoma growth in MEN1‑related cases. Additionally, next‑generation sequencing is uncovering sporadic mutations (e.g., in the DAXX and ATRX genes) that could become therapeutic targets.
What is the primary cause of Zollinger-Ellison syndrome?
It is caused by gastrin‑secreting neuroendocrine tumors, called gastrinomas, most often located in the duodenum or pancreas.
How is hypergastrinemia confirmed?
A fasting gastrin level above 1000 pg/mL is highly suggestive, but the secretin stimulation test remains the definitive confirmatory test.
Can Zollinger-Ellison syndrome be cured?
If the tumor is localized and surgically resectable, cure is possible. For metastatic disease, control relies on acid suppression and systemic therapies.
Why is MEN1 testing recommended?
Because a significant minority of patients have germline MEN1 mutations, which predispose them to additional endocrine tumors that require surveillance.
What role do somatostatin analogues play?
They bind to somatostatin receptors on gastrinomas, inhibiting gastrin release and thereby reducing acid output.

Devendra Tripathi
October 21, 2025 AT 13:13All this hype about gastrinomas is overblown; most patients never need radical surgery. The author forgets that PPIs alone can keep acid in check for years.
Vivian Annastasia
October 28, 2025 AT 10:53Oh great, another deep‑dive into a disease that only a handful of specialists ever see. Because what the world really needed was more academic jargon about secretin tests. You’ve managed to turn a fairly straightforward endocrine issue into a labyrinth of acronyms that would make any intern’s head spin. Readers will now spend their weekend deciphering ERK1/2 MAPK cascades instead of actually helping patients. The emphasis on MEN1 is cute, but most clinicians will never encounter a germline mutation in their practice. And the checklist? Nice, but it reads like a to‑do list for a medical student on a coffee‑fueled all‑night cram session. Sure, high‑dose PPIs are the first line, but you could have mentioned that many patients self‑adjust doses without supervision. The discussion on somatostatin analogues feels tacked on, as if you needed a buzzword to fill space. Also, the table formatting looks like it was copy‑pasted from a 1990s textbook. The article could have mentioned the real‑world challenge of insurance approvals for everolimus. Let’s not forget that the secretin stimulation test is a pain to schedule in most community hospitals. The piece briefly touches on nutrient malabsorption, but doesn’t explore the dietary adjustments patients actually need. And the future directions? Targeting menin‑MLL sounds promising, yet you provide zero context on trial phases. Overall, you’ve succeeded in turning a concise clinical topic into a marathon reading session. Congrats on the word count, but maybe trim the fluff next time.
Jake Hayes
November 3, 2025 AT 19:39While your disdain is noted, the data clearly shows that uncontrolled hypergastrinemia leads to refractory ulcers and nutrient loss. The pathophysiology you dismissed is central to patient outcomes. Early intervention can prevent the cascade of complications.
Brandy Eichberger
November 9, 2025 AT 14:33Appreciate the thoroughness of this write‑up; the breakdown of diagnostic modalities is especially useful. I’d add that EUS remains the gold standard for detecting subcentimeter duodenal lesions. Also, the reminder to stop PPIs before measuring gastrin cannot be overstated. For anyone managing such patients, integrating genetic counseling for MEN1 is a wise move.
Eli Soler Caralt
November 14, 2025 AT 19:33Totally agree! 👍 The checklist is super handy, especially the part about stopping PPIs two weeks prior. It’s the kind of practical tip we need more of. 😂 Also, love the nod to emerging menin‑targeted therapies – future looks bright! 🌟
Kathrynne Krause
November 19, 2025 AT 10:39Bravo for the comprehensive overview! Your colorful language makes the complex signaling pathways feel accessible. The blend of molecular detail with bedside practicality is exactly what clinicians crave. Keep lighting up the page with that vibrant enthusiasm!
Chirag Muthoo
November 23, 2025 AT 11:53The article presents an accurate synthesis of current knowledge regarding gastrin‑secreting neuroendocrine tumours. It correctly emphasizes the necessity of discontinuing acid‑suppressive therapy prior to biochemical assessment. Moreover, the delineation of imaging strategies aligns with contemporary guidelines. The discussion of MEN1‑related pathogenesis is concise yet informative.
Giusto Madison
November 26, 2025 AT 23:13Spot on with the formal summary, but let’s not downplay the urgency of initiating high‑dose PPIs. Delays in acid suppression can precipitate bleeding ulcers. Also, surgical resection offers the only potential cure in localized disease, so early referral is key.
Xavier Lusky
November 29, 2025 AT 20:39Never trust the pharma‑driven hype around everolimus; it’s just another profit scheme. The real issue is that most clinicians are blinded by industry‑funded studies, ignoring the long‑term toxicity profiles. Keep your eyes open.
Kimberly Lloyd
December 2, 2025 AT 04:13Reading this feels like witnessing the intricate dance of hormones and receptors-a reminder that biology is poetry in motion. Even in rare syndromes, there’s a lesson about balance and vigilance. May we all stay curious and compassionate.
Sakib Shaikh
December 3, 2025 AT 21:53Allow me to dramatize the scene: a once‑quiet stomach now erupts in a storm of acid, a silent villain-gastrinoma-lurking beneath the duodenum, plotting its relentless conquest. Yet, armed with PPIs and surgical scalpel, we become the heroic protagonists. This saga epitomizes the clash of science and disease.
John Price
December 5, 2025 AT 01:39Interesting read.
Nick M
December 5, 2025 AT 23:53Honestly, the whole “secretin stimulation test” is just a bureaucratic nightmare engineered by the diagnostic industry. They push these costly procedures while the real solution-patient education and lifestyle adjustment-gets sidelined. Stay skeptical.
eric smith
December 6, 2025 AT 16:33Wow, thanks for the endless footnotes. Because I was totally clueless about gastrin receptors. Guess we’ll all sleep better now knowing the exact MAPK cascade involved.