Have you ever picked up a prescription and noticed the pill looks exactly like your usual brand-name drug-but the box says something completely different? No logo. No fancy name. Just the generic chemical on the label? That’s probably an authorized generic.
It’s not a trick. It’s not a mistake. And it’s not the same as a regular generic drug. Authorized generics are the exact same medication as the brand-name version, made by the same company, in the same factory, with the same ingredients-except they’re sold without the brand name. They’re cheaper than the brand, but often more expensive than other generics. And they’ve been quietly changing how drugs are priced and sold since the 1990s.
What Exactly Is an Authorized Generic?
An authorized generic is a brand-name drug that’s sold under a different label-no trademark, no marketing, no fancy packaging. The FDA defines it clearly: it’s identical to the brand-name drug in every way. Same active ingredient. Same inactive ingredients. Same size, shape, color, and how it’s made. The only difference? It doesn’t carry the brand name.
Think of it like buying a Coca-Cola that’s bottled by Coca-Cola but sold under a store brand. It’s the exact same liquid, same recipe, same factory-but you’re not paying for the logo. That’s what an authorized generic is.
Unlike traditional generics, which must prove they work the same way through a process called bioequivalence testing (ANDA), authorized generics don’t need to go through that. Why? Because they’re already approved under the original brand’s New Drug Application (NDA). The FDA only needs to be notified, not re-approved. That means they hit the market faster and with zero uncertainty about how they perform.
How Are Authorized Generics Different from Regular Generics?
This is where things get confusing. Most people think all generics are the same. They’re not.
Traditional generics are made by other companies. They use the same active ingredient as the brand, but they can-and often do-use different fillers, dyes, or coatings. That’s allowed by the FDA as long as the drug works the same. But those differences can matter. Some patients report stomach upset, changes in how quickly the pill dissolves, or even different side effects when switching between generic versions.
Authorized generics don’t have that problem. They’re made by the original manufacturer using the exact same formula. No changes. No substitutions. If your brand-name drug worked perfectly for you, the authorized generic will too.
Here’s a quick comparison:
| Feature | Authorized Generic | Traditional Generic |
|---|---|---|
| Manufacturer | Brand-name company or its subsidiary | Separate generic drug maker |
| Active Ingredient | Identical to brand | Identical to brand |
| Inactive Ingredients | Identical to brand | May differ |
| Approval Process | Uses brand’s NDA; FDA notification only | ANDA required; must prove bioequivalence |
| Listed in FDA Orange Book? | No | Yes |
| Typical Price | 15-30% lower than brand | Often 70-90% lower than brand |
So if you’re someone who’s sensitive to small changes in medication, an authorized generic might be the safest switch you can make. No guesswork. No trial and error.
Why Do Brand Companies Make Authorized Generics?
It sounds strange. Why would a company that spent millions developing a drug turn around and sell a cheaper version of it?
The answer? Business strategy.
When a brand-name drug’s patent expires, other companies can legally make generics. But those generics usually come in fast and cheap. The brand company’s sales can drop by 80% in months. To protect their revenue, many manufacturers launch their own authorized generic-right before or right after the first generic hits the market.
Studies show that 75% of authorized generics launch after traditional generics have already entered. That’s not an accident. It’s a tactic. By offering their own version at a lower price, they capture the price-sensitive customers before the cheaper generics can take over.
It’s like a store selling its own store-brand version of its popular product to keep you from switching to a competitor’s version. They’re not trying to hurt themselves-they’re trying to control the transition.
Some experts call this a “defensive” move. Others call it a way to delay real competition. Either way, it’s legal, common, and growing. Between 2010 and 2019, over 850 authorized generics were launched in the U.S. alone.
Examples You Might Know
You’ve probably taken one without realizing it. Here are a few well-known examples:
- Colcrys (brand) → Colchicine (authorized generic by Prasco)
- Concerta (brand) → Methylphenidate ER (authorized generic by Watson/Actavis)
- Celebrex (brand) → Celecoxib (authorized generic by Greenstone)
- Unithroid (brand) → Levothyroxine (authorized generic by Jerome Stevens)
These aren’t obscure drugs. They’re used for gout, ADHD, arthritis, and thyroid conditions-conditions millions of people manage daily. If you’re on any of these, ask your pharmacist: Is this the authorized version?
Are Authorized Generics Cheaper?
Yes-but not always as cheap as you’d expect.
Authorized generics usually cost 15% to 30% less than the brand-name version. That’s a real savings. But once other generic manufacturers enter the market, prices can drop even further-sometimes to 90% below the brand.
So here’s the timing game: If you switch to an authorized generic right after patent expiry, you might save money immediately. But if you wait a few months, you might find a traditional generic for even less.
That’s why it pays to check your pharmacy’s pricing. Some insurers cover authorized generics at the same rate as traditional generics. Others don’t. Ask your pharmacist to compare the cash price of the brand, the authorized generic, and the other generics before you fill your prescription.
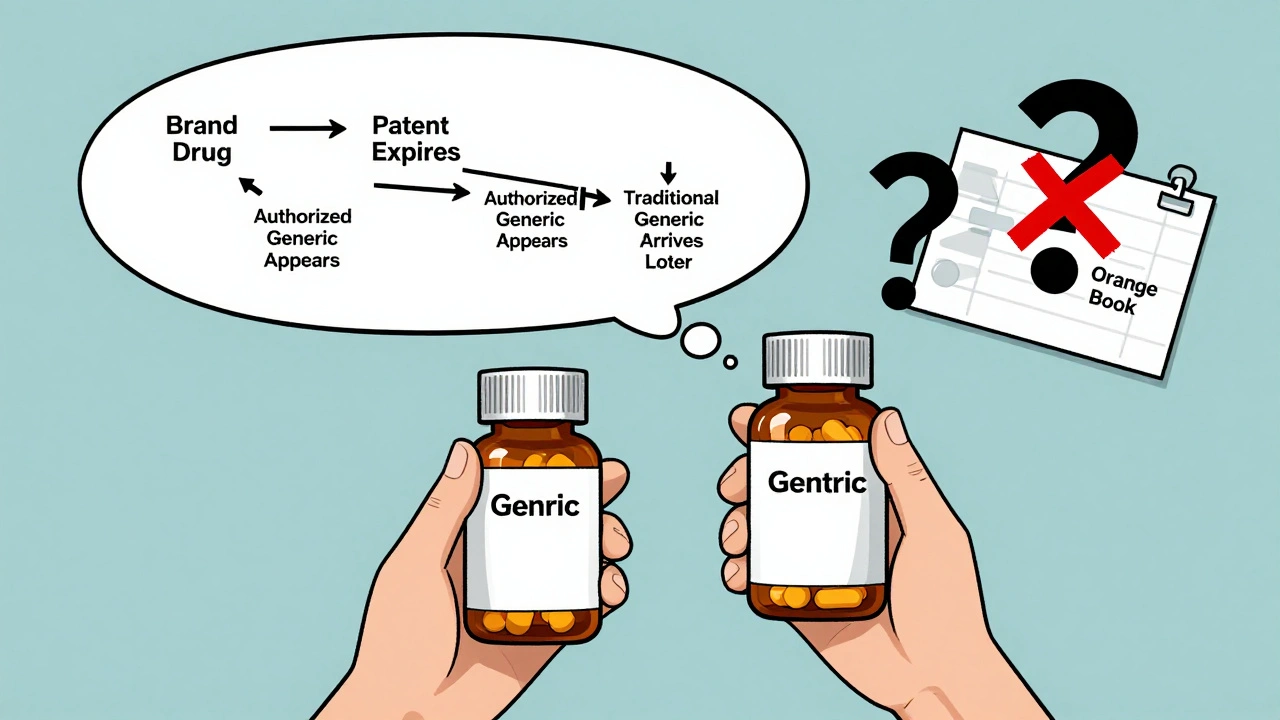
Why Aren’t They Listed in the FDA’s Orange Book?
The FDA’s Orange Book is the official list of approved generic drugs and their therapeutic equivalence ratings. But authorized generics? They’re not on it.
That’s because they’re not approved as generics. They’re approved as the brand drug-just sold under a different label. So the FDA doesn’t list them alongside traditional generics.
This creates a real headache for pharmacists and prescribers. If you’re looking up a drug in the Orange Book, you won’t see the authorized version. You have to know it exists and ask for it by name.
That’s why it’s smart to keep a list of your medications and their authorized generic equivalents. If your doctor prescribes a brand, you can ask: “Is there an authorized generic for this?”
What Should Patients Do?
Here’s what you need to know:
- Authorized generics are safe. They’re the exact same drug as your brand.
- They’re often cheaper than the brand, but not always the cheapest option.
- If you’ve had problems switching to traditional generics (side effects, lack of effectiveness), try an authorized generic first.
- Ask your pharmacist: “Is this an authorized generic?” and “Is there a cheaper generic available?”
- Don’t assume your insurance will cover it the same way as a traditional generic. Check your plan.
Some patients feel confused when they get a pill that looks exactly like their brand but costs less. That’s normal. The key is understanding: it’s not a downgrade. It’s the same medicine, just without the marketing.
What’s the Future of Authorized Generics?
They’re not going away. In fact, they’re likely to grow.
As more brand-name drugs lose patent protection, manufacturers will keep using authorized generics as a way to hold onto market share. Regulators are watching. Some lawmakers have questioned whether this practice delays true competition and keeps prices artificially high.
But for now, authorized generics remain a legal, common, and useful tool for patients who want a reliable, low-cost alternative to brand-name drugs-without the risk of formulation changes.
If you’re on a long-term medication, especially one that’s critical to your health, knowing about authorized generics could save you money-and maybe even prevent side effects from switching to a traditional generic with different inactive ingredients.
Are authorized generics as safe as brand-name drugs?
Yes. Authorized generics are made by the same company, in the same facility, with the exact same ingredients as the brand-name drug. The FDA considers them identical in safety, effectiveness, and quality. The only difference is the label.
Why do authorized generics cost more than regular generics?
Because they’re produced by the brand-name manufacturer, who doesn’t have the same cost-cutting pressures as independent generic makers. Traditional generics often compete fiercely on price, driving costs down. Authorized generics offer a middle ground: lower than brand, but not always the lowest price available.
Can I ask my pharmacist for an authorized generic by name?
Absolutely. Pharmacists can usually tell you if an authorized generic is available for your prescription. Just ask: “Is there an authorized generic for [drug name]?” They can check their inventory and pricing, and may even be able to switch your prescription if it’s allowed by your doctor.
Do authorized generics show up in my insurance formulary?
Sometimes. Insurance plans often group authorized generics with traditional generics, but not always. Some plans treat them like brand-name drugs for cost-sharing purposes. Always check your plan’s drug list or call your insurer to confirm coverage and your out-of-pocket cost.
How do I know if I’m getting an authorized generic?
Look at the label. If it lists the generic name (like “metformin”) and not a brand name (like “Glucophage”), but the pill looks identical to your previous brand, it might be an authorized generic. Ask your pharmacist to confirm. You can also check the FDA’s official list of authorized generics, updated as of October 2025.
Next Steps for Patients
Start by reviewing your current prescriptions. If you’re taking a brand-name drug that’s been on the market for more than 5 years, there’s a good chance an authorized generic exists. Talk to your pharmacist. Ask for a price comparison between the brand, the authorized generic, and any other generics.
If you’ve ever switched to a generic and felt “off,” consider asking for the authorized version next time. It’s the closest thing to staying on your brand-without the high price tag.
Knowledge is power. In a system full of confusing labels and hidden pricing, knowing what an authorized generic is-and how to get it-can save you money and keep your treatment consistent.


Ella van Rij
December 2, 2025 AT 23:24So let me get this straight - the pharma companies are selling their OWN drug under a different label to ‘compete’ with generics? Wow. So it’s not capitalism, it’s just… corporate theater with a side of patient confusion. 🤦♀️
Lynn Steiner
December 3, 2025 AT 00:16I hate this. I switched to a generic and my anxiety went through the roof. Then I found out my ‘generic’ was actually an authorized one - same pill, same factory. I cried. Not because I saved money. Because I realized I was manipulated for YEARS.
Alicia Marks
December 4, 2025 AT 23:15This is such a helpful breakdown! If you’ve ever felt ‘off’ after switching meds, try asking for the authorized version. It’s not magic - it’s just consistency. 💪
Joel Deang
December 5, 2025 AT 04:44wait so u mean like… the same pill but no logo?? 😳 i thought all generics were different… my bad lol
Roger Leiton
December 6, 2025 AT 18:46This changed my life. I’ve been on levothyroxine for 8 years. Switched to the authorized generic and my TSH stabilized within 2 weeks. No more brain fog. No more crashes. Just… me. 🙌
Shannara Jenkins
December 8, 2025 AT 09:19My pharmacist actually told me about this last month. I didn’t even know it was a thing. Now I always ask: ‘Is this the brand’s own generic?’ She laughs and says I’m her favorite patient. 😊
dave nevogt
December 9, 2025 AT 12:11There’s a profound irony here. The pharmaceutical industry, built on innovation and intellectual property, now relies on a legal loophole to preserve profit by offering the exact same product under a different label - a product that, by definition, requires no additional research, no clinical trials, no development cost. The consumer is left navigating a labyrinth of labels, pricing tiers, and regulatory obscurity, while the corporation maintains control over both the original and its shadow. This isn’t competition - it’s a controlled demolition of market disruption, orchestrated by the very entity that was supposed to be displaced. We’ve turned healthcare into a corporate chess game, and the patient is the pawn who doesn’t even know the board has been rearranged.
Elizabeth Grace
December 11, 2025 AT 07:48OMG I just checked my last prescription and it’s an authorized generic. I thought I was getting ripped off because it cost $12 instead of $8… but now I get it. It’s the same pill. I feel so dumb. And also… kinda relieved? 😅
Steve Enck
December 12, 2025 AT 00:06Let us not mistake this for consumer empowerment. The authorized generic is a predatory mechanism designed to suppress price elasticity by artificially maintaining a higher price floor than would otherwise exist under pure generic competition. It is a regulatory arbitrage - exploiting the FDA’s procedural distinction between NDA and ANDA to maintain market share without delivering proportional value. This is not innovation. It is rent-seeking disguised as patient care.
मनोज कुमार
December 12, 2025 AT 01:22authorized generic = same drug same factory cheaper than brand but not cheapest. why care? just take the 5 dollar one. problem solved. no need for all this drama.
Laura Baur
December 12, 2025 AT 11:19It’s not just about price - it’s about trust. When you’ve spent years on a medication and your body adapts to its exact formulation, the idea that a ‘generic’ could have different fillers that trigger migraines or GI distress isn’t anecdotal - it’s physiological. The authorized generic isn’t a compromise. It’s the only ethical option for patients who’ve been failed by the generic system’s ‘close enough’ philosophy. If your doctor prescribes a brand, you’re not being ‘expensive’ - you’re being responsible. And if your pharmacist doesn’t know this, they’re not doing their job.
Jack Dao
December 14, 2025 AT 07:14People who switch to authorized generics are just playing into Big Pharma’s hands. You think you’re saving money? You’re just delaying the inevitable - real competition. The real heroes are the ones who take the 80% cheaper generics and force prices down. Stop being sheep. 💪
Paul Keller
December 15, 2025 AT 20:33As someone who’s been on long-term psychiatric medication for over a decade, I can say with absolute certainty: authorized generics saved my stability. I switched from brand to a traditional generic twice - both times I developed tremors and insomnia. The third time, I asked for the authorized version. No side effects. No guesswork. Same pill. Same factory. Same me. The system is broken, but this is one of the few legal, safe, and genuinely patient-centered workarounds. If you’re on a chronic med, don’t assume ‘generic’ means ‘identical.’ Ask. Always ask.