
Generic drugs are evolving from low-cost copies to high-stakes biotech products. Discover how biosimilars, supply chain shifts, and emerging markets will reshape global healthcare access by 2030.
read more
AI and pharmacogenomics are transforming online pharmacies by delivering personalized generic drug recommendations based on your DNA. Learn how it works, who’s using it, and why it could prevent dangerous side effects.
read more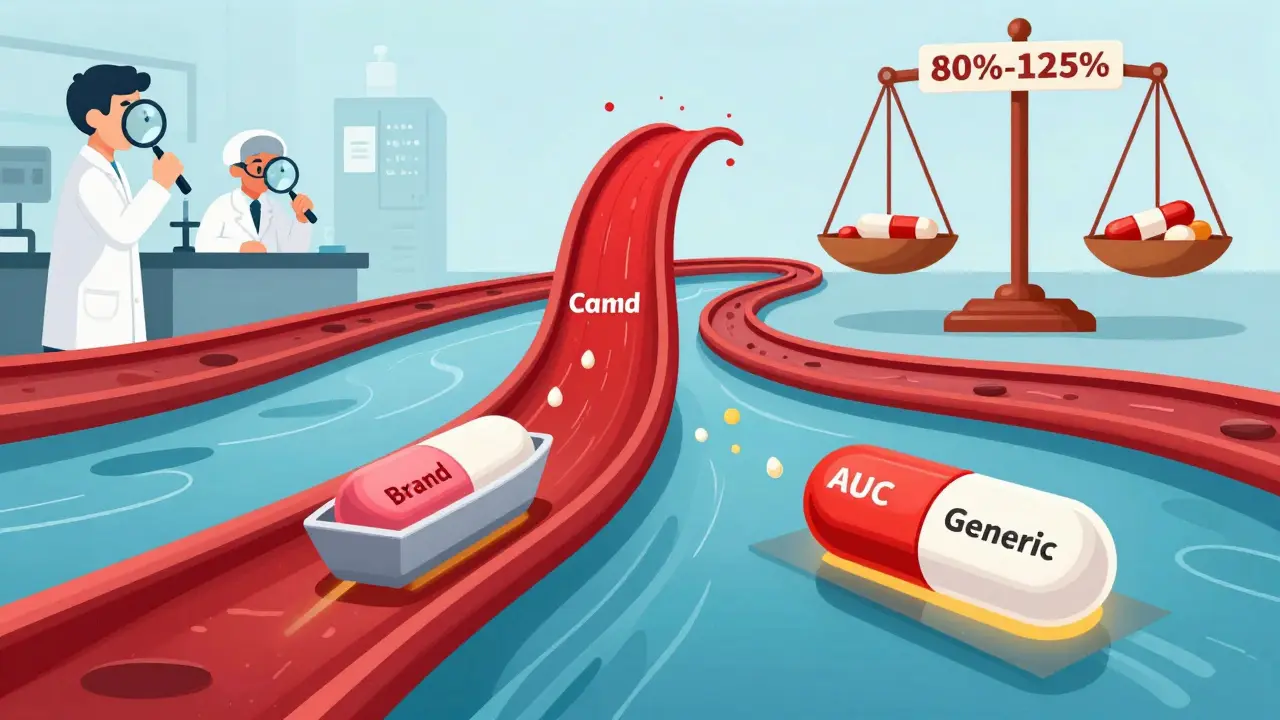
Cmax and AUC are the two key pharmacokinetic measures used to prove generic drugs work the same as brand-name versions. They track peak concentration and total exposure - and both must fall within strict limits to ensure safety and effectiveness.
read more
Paragraph IV certifications let generic drug makers challenge brand patents before launching. This legal tool under the Hatch-Waxman Act has saved U.S. consumers over $2 trillion since 1984 by speeding up generic access.
read more
Switching from brand to generic medication saves money without sacrificing effectiveness for most people. Learn what to expect, when to be cautious, and how to stay safe when making the switch.
read more
Authorized generics are brand-name drugs sold without the brand name, made by the same company with identical ingredients. They offer a middle-ground option between expensive brands and cheaper traditional generics, with no formulation changes.
read more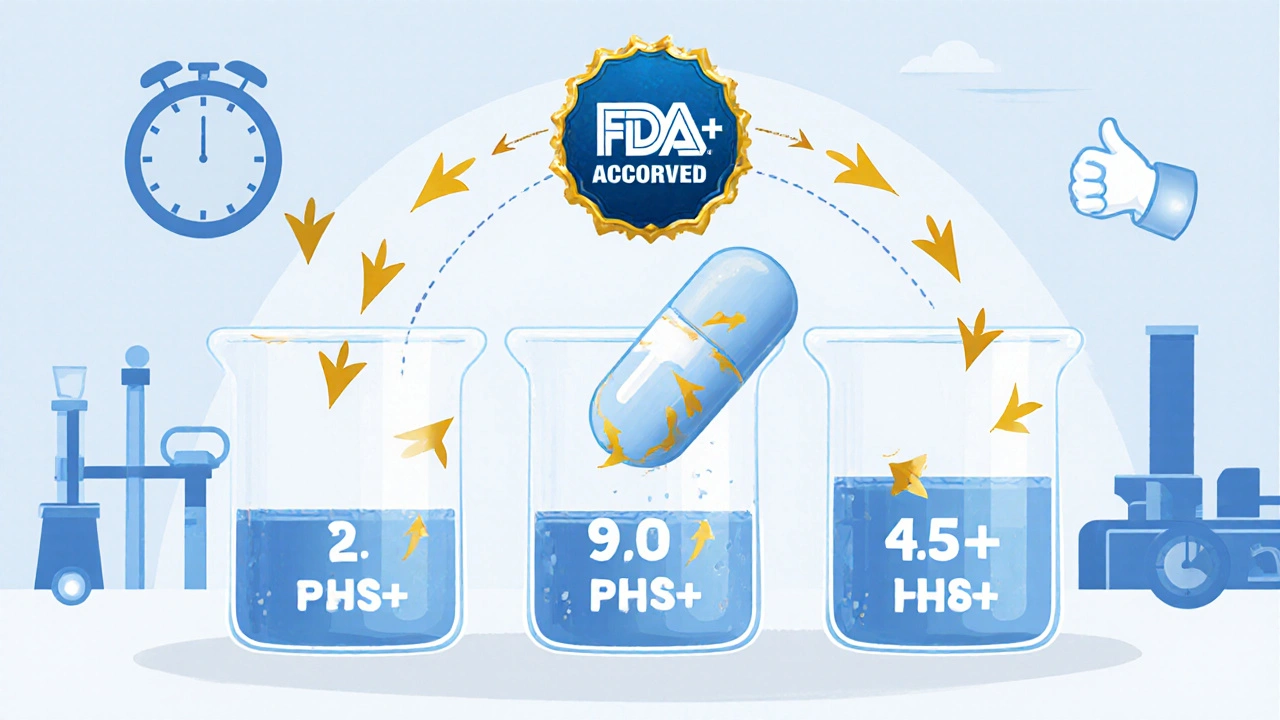
The FDA allows bioequivalence waivers for certain generic drugs when in vitro dissolution data proves they perform like brand-name versions. This saves time and money while ensuring safety - but only for drugs meeting strict BCS Class I or III criteria.
read more